About
Sue Gibbs is professor in Skin and Mucosa Regenerative Medicine. She is PI at the Department of Molecular Cell Biology & Immunology and also in the Department of Oral Cell Biology, ACTA. Her entire career has focused on animal alternative methods, in particular in developing human healthy and disease models drug discovery, pre-clinical studies and safety testing. She combines cutting-edge research in cell biology and immunology with advances in tissue engineering. Current focus lies with developing next generation immune competent tissue engineered constructs to investigate the (patho)physiology of adverse scar formation and to investigate similarities and differences between tolerance, allergic and irritant contact dermatitis with the aim of identifying novel drug targets for personalized as well as general therapeutic strategies. Recently, her research has extended into the field of hair follicles and importantly ‘’organ-on-a-chip’’, in particular immune competent skin-, gingiva-, gut,- lymph node- and melanoma- “on-chip”. Sue Gibbs was awarded numerous grants from EU, NWO, TTW, Dutch Burns foundation, in collaboration with partners from industry.
Research Line
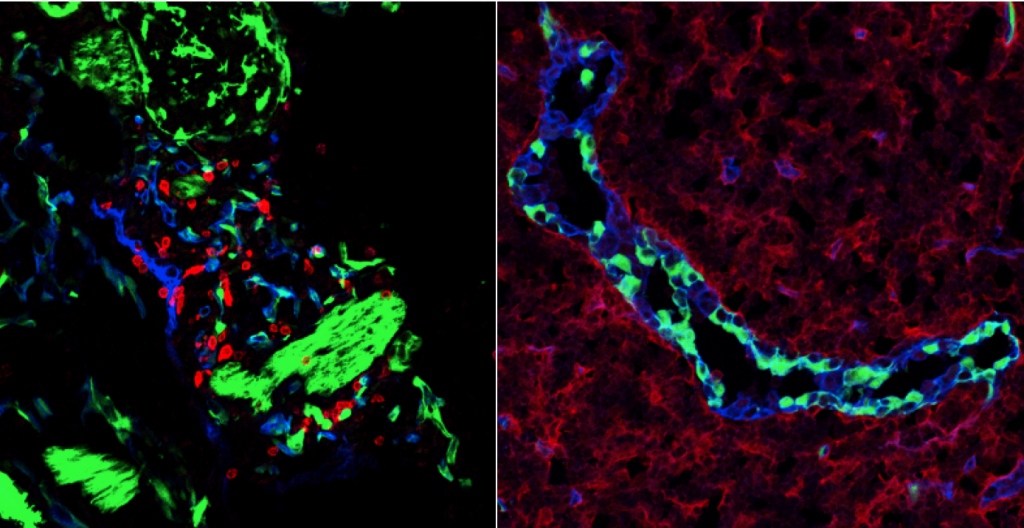
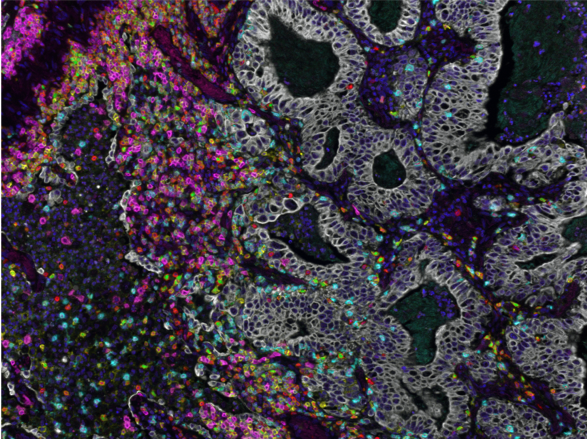
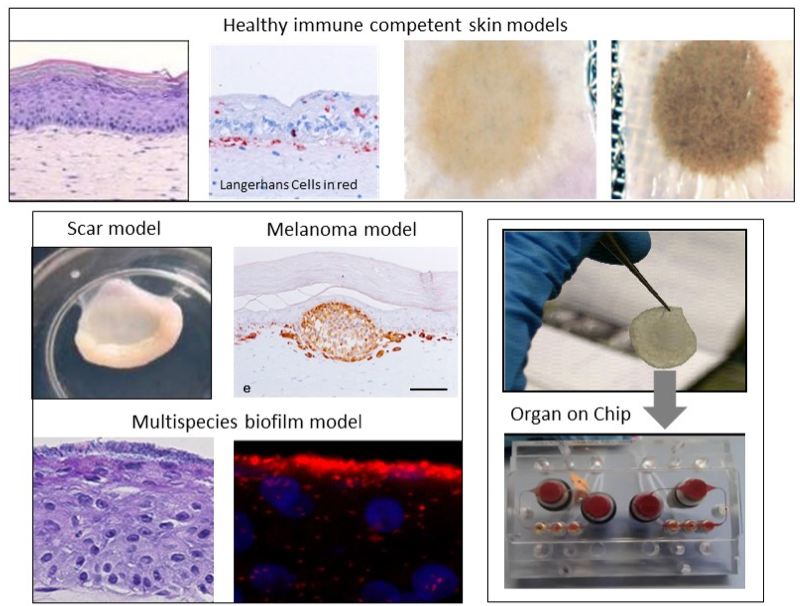
Our aim is to develop the next generation immune competent human skin models which closely represent healthy and diseased skin, thus providing a platform for safety and efficacy testing of drugs and consumer products which come into contact with our skin. Our focus is on fibrosis, cancer, allergy and tolerance. Cell types which can currently be built into our organotypic models include epithelial cells (keratinocytes, melanocytes, Langerhans cells, melanoma), dermal cells (fibroblasts, myofibroblasts, hair follicles, blood and lymph vessel endothelial cells, dendritic cells, monocytes, macrophages) and adipose tissue cells (mesenchymal stromal cells, adipocytes and endothelial cells). These models are being incorporated into micro-physiological bioreactors, ‘’organ-on-a-chip’’, to create immune competent skin-, gingiva-, gut,- lymph node- and melanoma- “on-chip”.
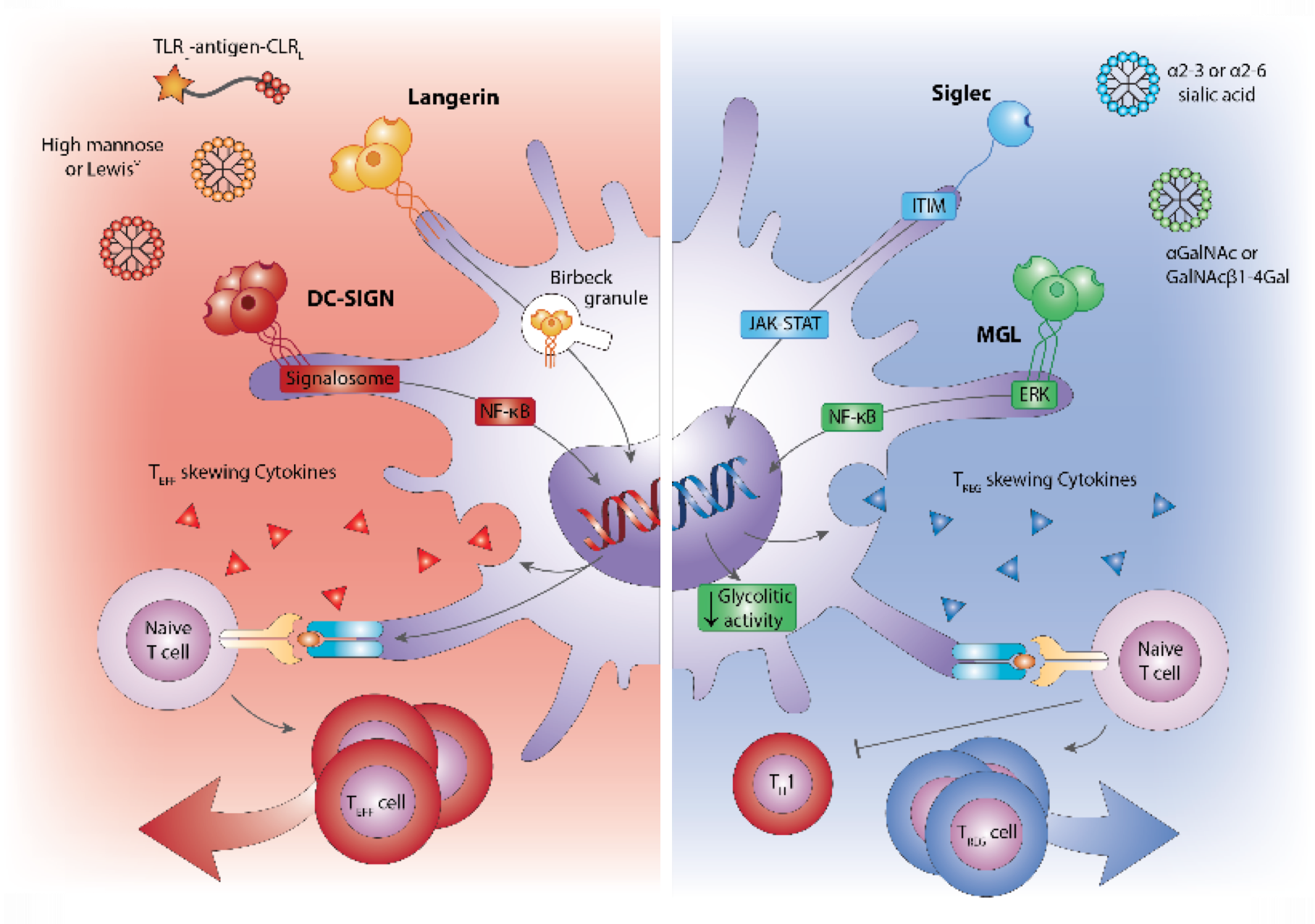
Research is aimed at identifying the mechanisms involved in skin and oral soft tissue homeostasis and inflammation. Examples of our research questions include: why does a deep skin wound heal with a bad scar formation whilst oral wounds heal with relatively little scar? Is this to do with intrinsic properties of the cells or is this due to extrinsic factors e.g.: saliva, microbiome? With the aid of normal skin / gingival equivalents, and in vitro hypertrophic scar and keloid scar equivalents the mechanisms of scar formation can be investigated. Another major research question is: why does first contact with an allergen (e.g. Nickel) via the skin often result in sensitization whereas first contact via the mouth with the same allergen results in tolerance? With the aid of skin and gingival equivalents containing integrated Langerhans Cells, innate and adaptive mechanisms involved in sensitization versus tolerance and pathogen infection are being investigated.
Key publications
- Koning JJ, et al.. A Multi-Organ-on-Chip Approach to Investigate How Oral Exposure to Metals Can Cause Systemic Toxicity Leading to Langerhans Cell Activation in Skin. Front Toxicol. 2022. 15;3:824825.
- A Reconstructed Human Melanoma-in-Skin Model to Study Immune Modulatory and Angiogenic Mechanisms Facilitating Initial Melanoma Growth and Invasion. Michielon E, López González M, Stolk DA, Stolwijk JGC, Roffel S, Waaijman T, Lougheed SM, de Gruijl TD, Gibbs S.Cancers (Basel). 2023 May 20;15(10):2849. doi: 10.3390/cancers1510284
- Shang, L., et al., Commensal and Pathogenic Biofilms Alter Toll-Like Receptor Signaling in Reconstructed Human Gingiva.Front Cell Infect Microbiol. 2019 Aug 7;9:282. doi: 10.3389/fcimb.2019.00282. eCollection 2019
- Vahav, I., et al., Reconstructed human skin shows epidermal invagination towards integrated neopapillae indicating early hair follicle formation in vitro. J Tissue Eng Regen Med. doi:10.1002/term.3039 2020
- Kosten, I.J., et al., MUTZ-3 derived Langerhans cells in human skin equivalents show differential migration and phenotypic plasticity after allergen or irritant exposure. Appl. Pharmacol, 2015. 287(1): p. 35-42.
Group members

Andrew Morrison, MSc
PhD student
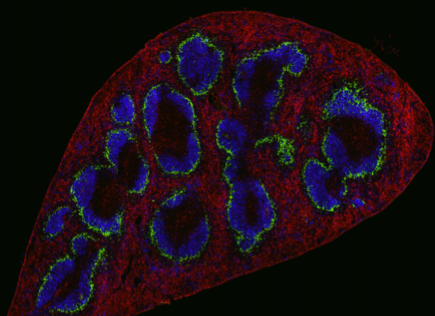
My project aims to develop a functional 3D organotypic lymph node with integrated lymphatics that mimic adaptive immune responses. Combining research on lymph node stromal cells and current in-house expertise of tissue engineering & microfluidic devices, the overall objective of the project is to design an organ-on-chip model that features lymphatic drainage of an immune competent gut into a lymph node. The immunosurveillance capability of the model will then be assessed by inducing an inflammatory response, leading to its availability as a future platform for rheumatoid arthritis drug testing.

Elisabetta Michielon, MSc
PhD student
My project focuses on developing an organotypic three-dimensional in vitro metastatic melanoma-in-skin model to study disease development and progression, as well as in situ tumor-induced immune suppression. The incorporation of immune cells into the model and its further integration into a microfluidic device will provide a valuable tool for the testing of immunotherapies in a relevant human setting. To this aim and to unravel features of the melanoma model, I mostly use tissue engineering in combination with cytokine ELISAs, immunohistochemistry, and flow cytometry.

H. Ibrahim Korkmaz, PhD
Postdoctoral researcher
Treating patients with severe burns and understanding the mechanisms involved, which can lead to improved treatment strategies, is very complex. The aim of my project is the development, optimization and standardization of a 3D (printed) reconstructed human skin model for burn wound healing.
Moreover, I am working on a totally new approach which is complementary to organotypic skin models which are used to investigate wound healing and fibrosis: it is a systems biology approach to understanding burn wound healing.

Jasper Koning, PhD
Postdoctoral researcher
My works focuses on developing complex in vitro human metabolically and immune competent skin model connected to microfluidics vasculature in a skin on a chip format. This model can be used for experimental research, testing of compounds as well as drug discovery.

Jonas Jäger, MSc
PhD student
• I have a strong interest in tissue engineering. We are constructing metabolically active and immune competent skin equivalents with the aim to reduce animal testing for the safety assessment of drugs and chemicals. Therefore, we use multi-organ-chips (or so called microphysiological systems), bioprinting and various analysis methods such as immunohistochemistry, RNA sequencing, cytokine ELISAs, (3D-)microscopy and toxicity assays.

Lin Shang, PhD
postdoctoral researcher
My project is about developing a host-microbe interaction model using reconstructed human gingiva and multi-species oral biofilms to mimic the native situation in the oral cavity. With this model we aim to study how the oral host-microbe interactions correlate with oral health and disease, using techniques such as (immune)histochemical staining, ELISAs, 16S rRNA sequencing, metabolic assays.

Lisa Lee Bruske, MSc
PhD student

Caroline de Jongh, MSc
PhD student

Joey Karregat, MSc
PhD student

Britt van der Leeden, MSc
PhD student

Rico Balk, MSc
research technician

Maria Thon, Ing
senior research technician
My expertise is in culturing reconstructed human skin models for many different research questions. I am specialized in skin, hair, endothelial cell, adipose tissue isolation and culture. Detection techniques: IHC (also fluorescence), ELISA , WB, qPCR. I have experience with good laboratory practice (GLP), cleanroom production (GMP) of skin constructs for chronic wounds and burns, METC and Kwaliteitsnet. Currently I am working on the NextSkin project.

Melis Asal, MSc
PhD student
The focus of my work is developing an immune competent organotypic Gut-on-Chip model capable of immune surveillance. The model will be used for mimicking the effect of microbiome, testing immune responses and uptake of orally digested drugs for rheumatoid arthritis.

Sander W. Spiekstra, BSc
Operator / Senior technician
My entire career has focussed on the refinement and replacement of animal models to perform risk assessment on chemicals which may potentially cause an irritant or allergenic reaction in skin. The immune defence system of the skin has my greatest interest . As operator and specialist in the culturing of 3D immunocompetent skin equivalents with Langerhans Cells and Dendritic Cells, i am responsible for theoretical and technical assistance in these research lines . As one of the operators of the Attune flow cytometer and member of MCCF (microscopy and flow facility) i am happy to assist with flow cytometry experiments on this machine. (3D) Tissue culturing, Microscopy (light and fluorescence), Flow cytometry, RT-PCR (RNA profiling), protein arrays , Elisa, are techniques i use for my research. I am the “business information manager” for the statistical analysis program Graphpad. I am more than average trained to answer statistical questions. I serve as a contact person between the polyclinic Dermatology / Allergology in the location AMC and am trained in performing contract research for industrial partners. Also the maintenance of laboratory devices is one of my tasks in the lab.

Sanne Roffel, Ing
Research technician
I’m a research technician so have been working on a lot of different projects and still do.
Have a lot experience in culturing reconstructed human skin and gingiva models. These models are made with primary cells as well as with cell lines (ML II lab). These models are used for a wide spectrum of experiments. Like, wound healing questions properties, chemical exposures, implant attachment etc.
To analyze the cultures I can use a lot of different techniques like, IHC (also Fluorescence), hard tissue embedding, FISH, ELISA, FACS and PCR. But also have some knowledge of the analytical techniques: HPLC and CE.

Joline Boogaard, MSc
Research technician

Taco Waaijman, MSc
Research technician
My research focus on culturing of different kind of 3D skin- and gingiva-models, in combination with Organon-a-chip systems.
Other PI's
Febe van Maldegem
Gijs Kooij
Elga de Vries
Sandra van Vliet
Reina Mebius
Yvette van Kooyk
Jack van Horssen
Joke den Haan
Juan J. Garcia Vallejo
Marjolein van Egmond
Lorem ipsum dolor sit amet, consectetur adipiscing elit. Ut elit tellus, luctus nec ullamcorper mattis, pulvinar dapibus leo.
Lorem ipsum dolor sit amet, consectetur adipiscing elit. Ut elit tellus, luctus nec ullamcorper mattis, pulvinar dapibus leo.